
Neurobiology of Sleep and Wakefulness
This content was developed using literature published in peer-reviewed journals and other materials.
Neurobiology of Sleep and Wakefulness
This content was developed using literature published in peer-reviewed journals and other materials.
Overview
Like orexin (hypocretin) neurons, histamine neurons play an important role in promoting and stabilizing wakefulness.1-4


Exploring histamine in sleep and wakefulness
Watch for a more in-depth discussion about histamine, including key data from animal studies that support the importance of histamine in disorders characterized by sleep-wake state instability, such as narcolepsy.1,2
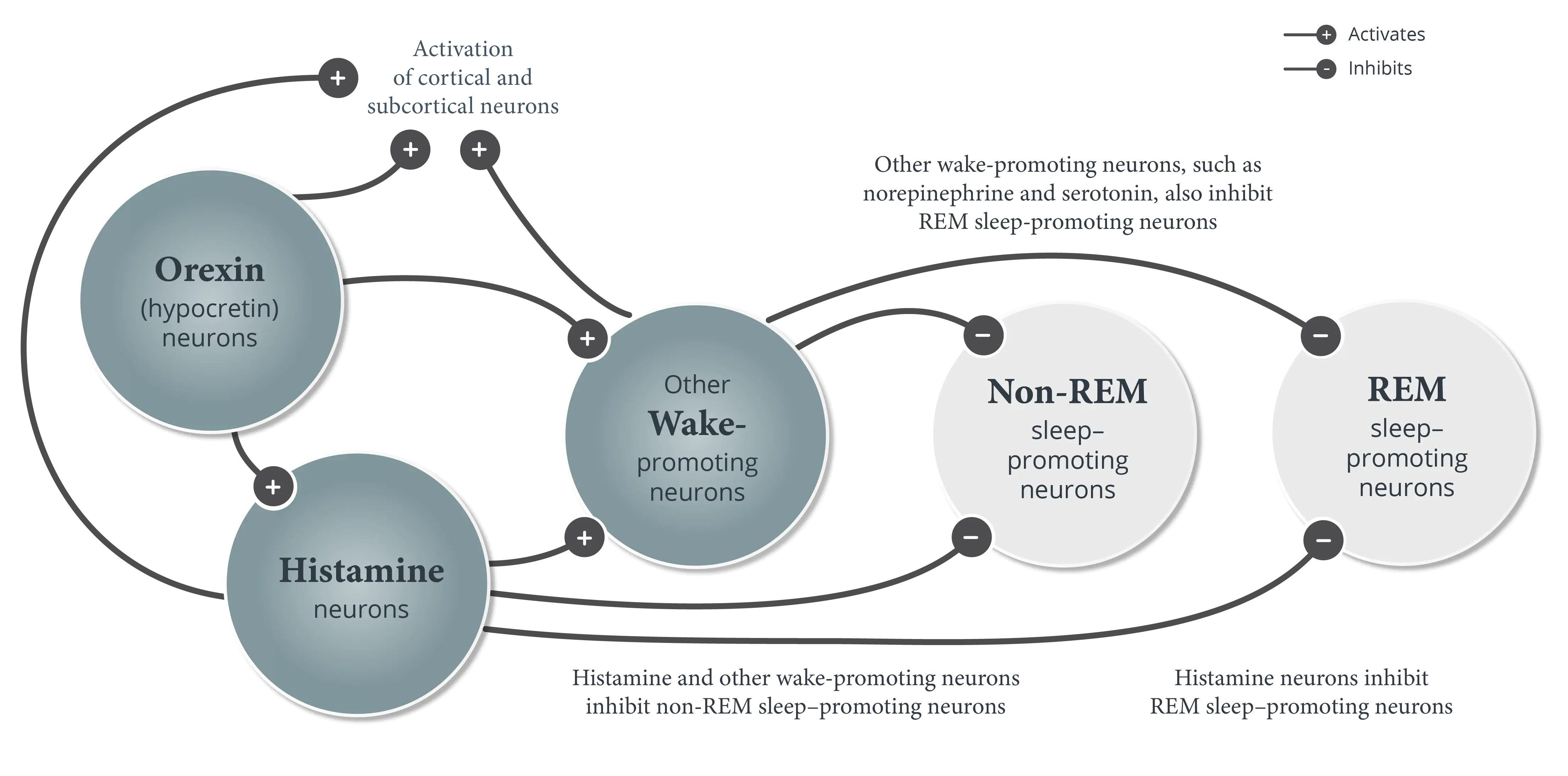
In normal wakefulness and sleep (non-REM and REM sleep), the boundaries between these states are stable, ensuring that elements from one state do not intrude into another state and transitions from one state to another are infrequent and predictable.1,5,6
The hypothalamus is a critical “control center” for sleep-wake state stability.* Several neuronal systems in the hypothalamus are responsible for the coordinated timing and appropriate duration of wakefulness, non-REM sleep, and REM sleep.5,7-9
These neuronal systems include:
Lateral hypothalamus (LH)1,5,9,10
- The LH is the only part of the brain where orexin (hypocretin) neurons originate5,11,12
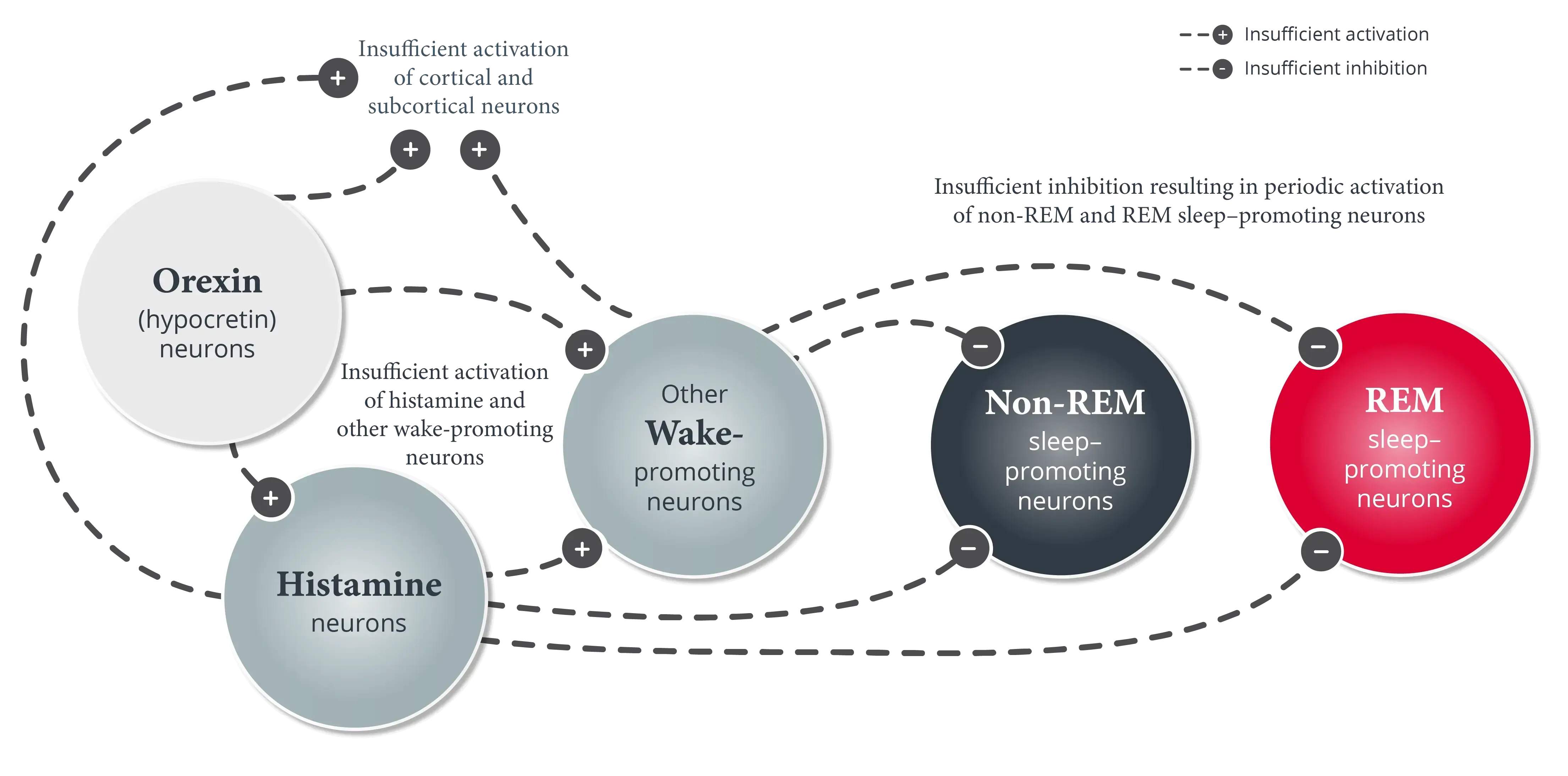
- Orexin (hypocretin) neurons promote wakefulness by activating cortical and subcortical neurons, such as histamine neurons and other wake-promoting neurons outside of the hypothalamus5,12,13
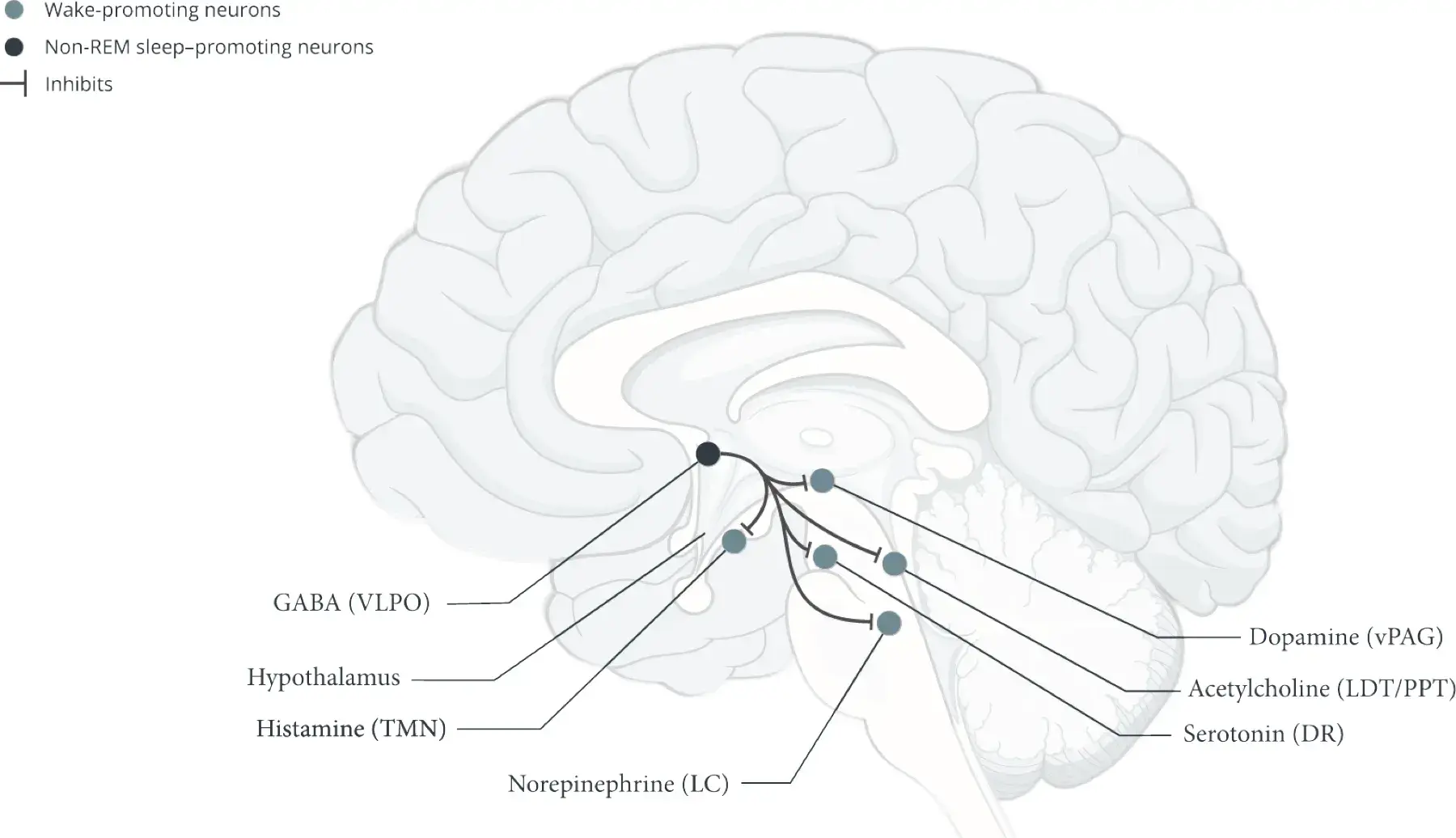
- Histamine and other wake-promoting neurons inhibit non-REM sleep–promoting neurons and REM sleep–promoting neurons.1,5 Orexin (hypocretin) neurons reinforce this activity to help stabilize wakefulness during the day1
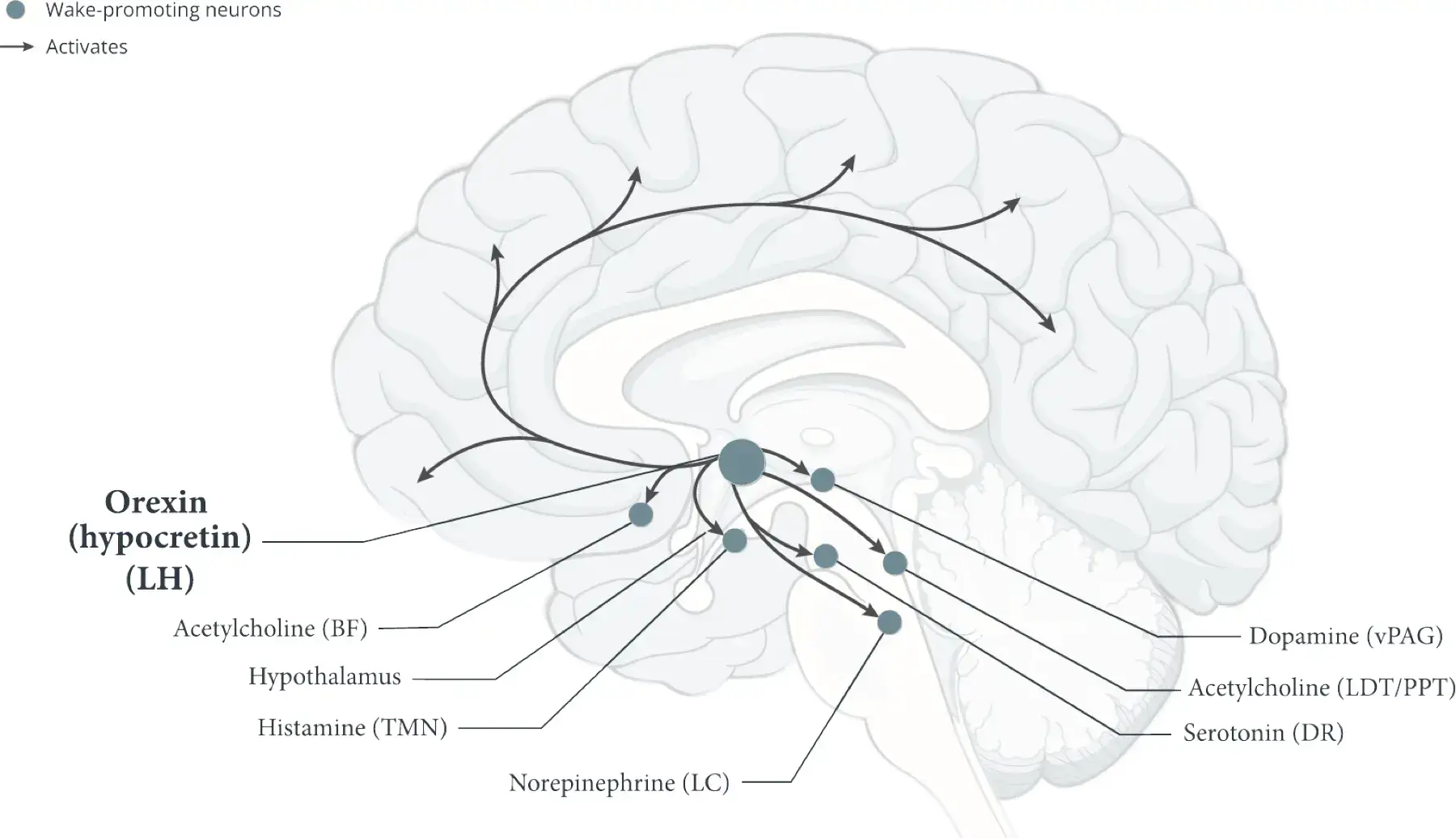

Tuberomammillary nucleus (TMN)1-3,5,14
- The TMN is the only neuronal source of histamine in the brain2,5
- Histamine neurons:
- Promote wakefulness by activating cortical and subcortical neurons, as well as wake-promoting neurons outside of the hypothalamus2
- Stabilize wakefulness by inhibiting non-REM sleep–promoting neurons and REM sleep–promoting neurons2,3,5,15
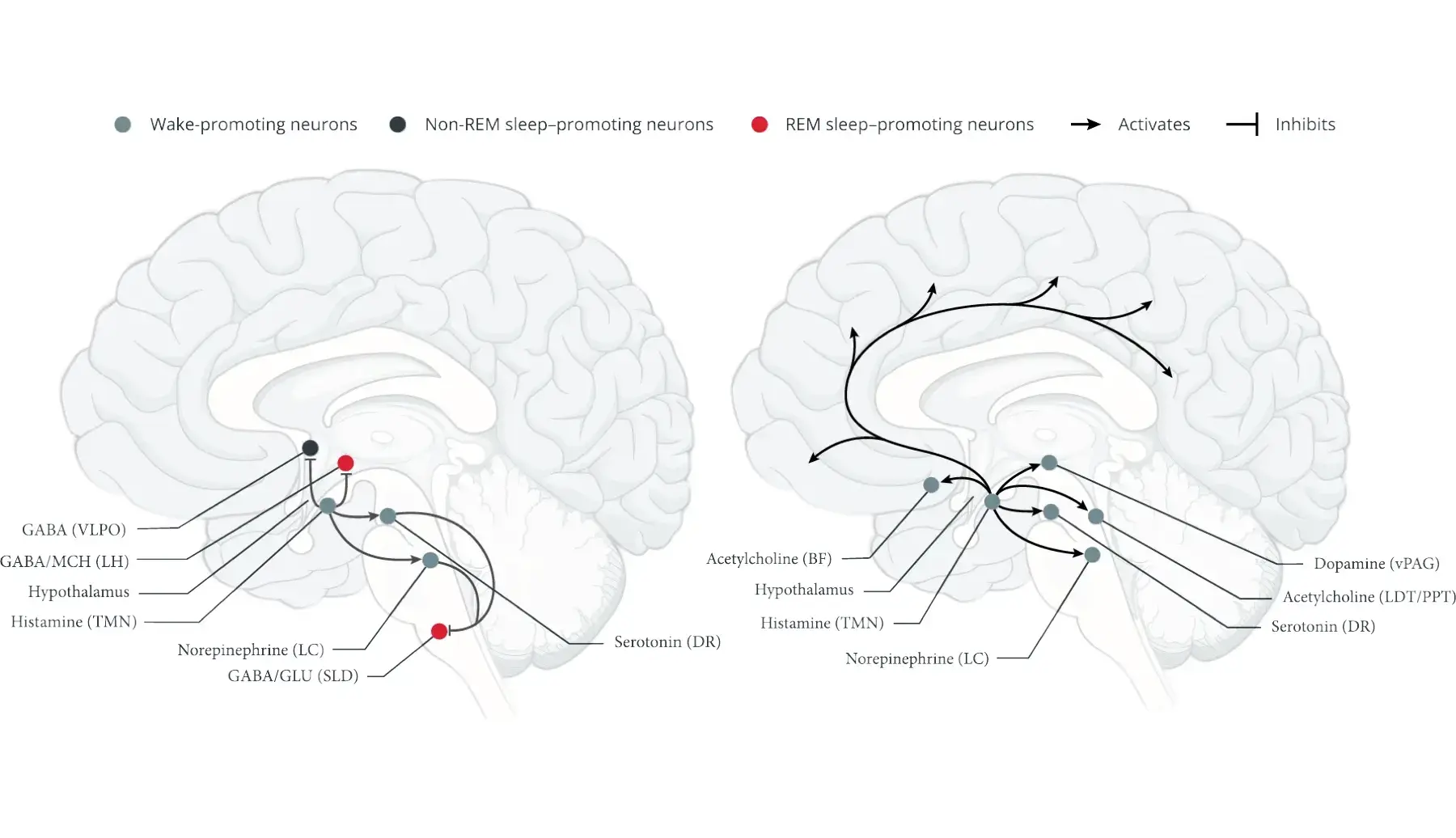

Ventrolateral preoptic area (VLPO)1,5,9
- The VLPO and the median preoptic nucleus contain essential neurons for promoting non-REM sleep1,5
- These neurons project to key wake-promoting regions in the brain to inhibit wakefulness
- Neurons in the extended VLPO promote REM sleep by inhibiting certain wake-promoting neurons that suppress REM sleep5

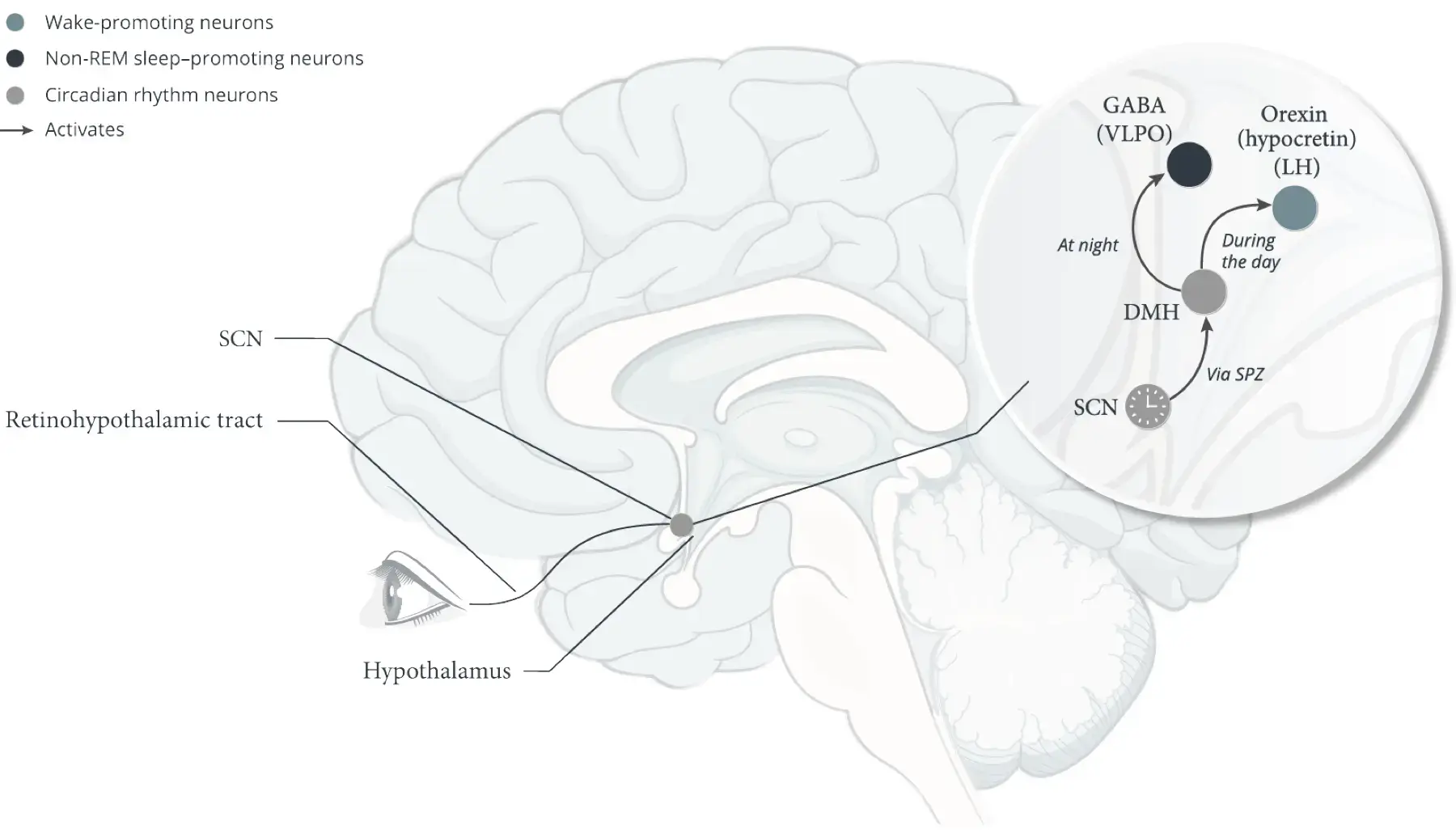
Suprachiasmatic nucleus (SCN)5,9,16,17
- The SCN coordinates circadian timing and other circadian rhythms to align sleep and wakefulness to the daily light/dark cycle5

Normal sleep and wakefulness require the coordinated activity of several neuronal systems in various regions of the brain.4,11
See how the loss of orexin (hypocretin) leads to unstable boundaries between sleep and wake states.1
*Based on animal and human studies.
BF, basal forebrain; DMH, dorsomedial hypothalamic nucleus; DR, dorsal raphe; GABA, gamma-aminobutyric acid; GLU, glutamate; LC, locus coeruleus; LDT, laterodorsal tegmentum; LH, lateral hypothalamus; MCH, melanin-concentrating hormone; PPT, pedunculopontine tegmentum; SCN, suprachiasmatic nucleus; SLD, sublaterodorsal nucleus; SPZ, subparaventricular zone; TMN, tuberomammillary nucleus; VLPO, ventrolateral preoptic area; vPAG, ventral periaqueductal gray.
References
- España RA, Scammell TE. Sleep neurobiology from a clinical perspective. Sleep. 2011;34(7):845-858.
- Haas HL, Sergeeva OA, Selbach O. Histamine in the nervous system. Physiol Rev. 2008;88(3):1183-1241.
- Scammell TE, Jackson AC, Franks NP, Wisden W, Dauvilliers Y. Histamine: neural circuits and new medications. Sleep. 2019;42(1):zsy183. doi:10.1093/sleep/zsy183
- Schwartz MD, Kilduff TS. The neurobiology of sleep and wakefulness. Psychiatr Clin North Am. 2015;38(4):615-644.
- Scammell TE, Arrigoni E, Lipton JO. Neural circuitry of wakefulness and sleep. Neuron. 2017;93(4):747-765.
- Broughton R, Valley V, Aguirre M, Roberts J, Suwalski W, Dunham W. Excessive daytime sleepiness and the pathophysiology of narcolepsy–cataplexy: a laboratory perspective. Sleep. 1986;9(1 pt 2):205-215.
- van der Heide A, Lammers GJ. Narcolepsy. In: Thorpy MJ, Billiard M, eds. Sleepiness: Causes, Consequences and Treatment. Cambridge University Press; 2011:111-125.
- Shan L, Dauvilliers Y, Siegel JM. Interactions of the histamine and hypocretin systems in CNS disorders. Nat Rev Neurol. 2015;11(7):401-413.
- Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257-1263.
- Pintwala SK, Peever J. Brain circuits underlying narcolepsy. Neuroscientist. 2021;29(6):751-766.
- Schwartz JRL, Roth T. Neurophysiology of sleep and wakefulness: basic science and clinical implications. Curr Neuropharmacol. 2008;6(4):367-378.
- Scammell TE. The neurobiology, diagnosis, and treatment of narcolepsy. Ann Neurol. 2003;53(2):154-166.
- Scammell TE. Narcolepsy. N Engl J Med. 2015;373(27):2654-2662.
- Thorpy MJ, Bogan RK. Update on the pharmacologic management of narcolepsy: mechanisms of action and clinical implications. Sleep Med. 2020;68:97-109.
- Williams RH, Chee MJS, Kroeger D, et al. Optogenetic-mediated release of histamine reveals distal and autoregulatory mechanisms for controlling arousal. J Neurosci. 2014;34(17):6023-6029.
- von Gall C. The effects of light and the circadian system on rhythmic brain function. Int J Mol Sci. 2022;23(5):2778. doi:10.3390/ijms23052778
- Makrygianni EA, Chrousos GP. Neural progenitor cells and the hypothalamus. Cells. 2023;12(14):1822. doi:10.3390/cells12141822